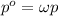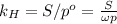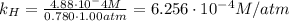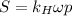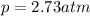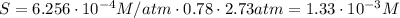Chemistry, 18.12.2019 04:31, terrysizemore666
Air is a mixture of gases that is about78.0percent n2 by volume. when air is at standard pressure and 25.0 degreec, the n2 component will dissolve in water with a solubility of 4.88 x 10^-4m. what is the value of henry's law constant for n2 under these conditions? express the constant numerically inmoles per liter per atmosphere. =6.26? 10^-4 mol/(l. atm) correct part b as a scuba diver descends under water, thepressure increases. at a total air pressure of 2.73 atm and a temperature of 25.0 degreec, what is the solubility of n2 in a diver's blood? [use the value of the henry's lawconstant calculated in part a, 6.26 x 10^-4mol/(l. atm). assume that the composition of the air in the tank is the sameas on land and that all of the dissolved nitrogen remains in theblood. express your answer numerically inmoles per liter. solubility =mol/l

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, catdog5225
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 22.06.2019 23:50, datboyjulio21
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1

Chemistry, 23.06.2019 07:00, SMURFETTE86
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2

Chemistry, 23.06.2019 08:20, debramknoxx
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
Do you know the correct answer?
Air is a mixture of gases that is about78.0percent n2 by volume. when air is at standard pressure an...
Questions in other subjects:

Business, 12.07.2019 22:30

Computers and Technology, 12.07.2019 22:30

Mathematics, 12.07.2019 22:30


Health, 12.07.2019 22:30



Social Studies, 12.07.2019 22:30

Social Studies, 12.07.2019 22:30

Social Studies, 12.07.2019 22:30


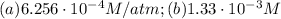
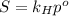
![p = 1.00 atm[/atm]Nitrogen's pecentage is:[tex]\omega = 0.780](/tpl/images/0423/6471/ab47c.png)