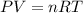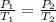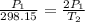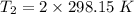Gsuppose a sample of an ideal gas in a container is subjected to a temperature change. a decrease in temperature will the kinetic energy and average speed of the gas particles. as a result, the pressure on the walls of the container will if the gas starts at 25 ∘ c, what temperature would the gas need to reach for its pressure to double? temperature = ∘ c

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
Do you know the correct answer?
Gsuppose a sample of an ideal gas in a container is subjected to a temperature change. a decrease in...
Questions in other subjects:


History, 01.08.2019 06:20



Mathematics, 01.08.2019 06:20




Biology, 01.08.2019 06:20