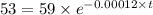The half life for the decay of carbon-14 is 5.73 x 10^3 years. suppose the activity due to the radioactive decay of the carbon-14 in a tiny sample of an artifact made of wood from an archeological dig is measured to be 53.bq. the activity in a similar-sized sample of fresh wood is measured to be 59.bq.
1. calculate the age of the artifact. round your answer to 2 significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 23.06.2019 04:31, CassidgTab
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3

Chemistry, 23.06.2019 05:00, daytonalive6511
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3

Chemistry, 23.06.2019 09:00, joelpimentel
The concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point
Answers: 3
Do you know the correct answer?
The half life for the decay of carbon-14 is 5.73 x 10^3 years. suppose the activity due to the radio...
Questions in other subjects:




Spanish, 01.07.2020 17:01


Mathematics, 01.07.2020 17:01





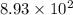 years
years
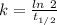
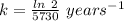
![[A_t]](/tpl/images/0423/2686/5262c.png) = 53 Bq
= 53 Bq![[A_t]=[A_0]e^{-kt}](/tpl/images/0423/2686/1ef89.png)