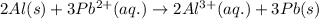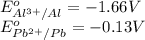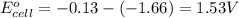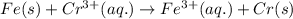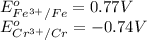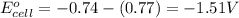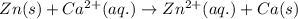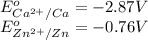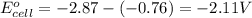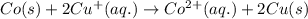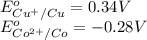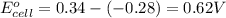Chemistry, 17.12.2019 23:31, melissacornut
Based on the reduction potentials listed in the textbook appendix, which of the following redox reactions do you expect to occur spontaneously?
w. 2al(s)+3pb2+ (aq) → 2al3+ (aq)+3pb(s)
x. fe(s)+cr3+ (aq) → fe3+ (aq)+cr(s)
y. ca2+ (aq)+zn(s) → ca(s)+zn2+(aq)
z. 2cu+(aq)+co(s) → 2cu(s)+co2+ (s)
a. w only
b. x, y and z
c. y only
d. x and z
e. z only
f. x and y
g. w, x and z
h. x and y
i. w and z

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 23.06.2019 01:30, Thunderalesis7855
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
Do you know the correct answer?
Based on the reduction potentials listed in the textbook appendix, which of the following redox reac...
Questions in other subjects:


Mathematics, 13.04.2020 21:19




Mathematics, 13.04.2020 21:19




Chemistry, 13.04.2020 21:19


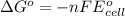
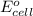 of the reaction, we use the equation:
of the reaction, we use the equation: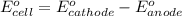 .......(1)
.......(1)