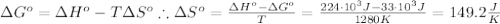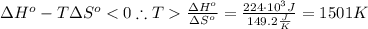The standard reaction enthalpy of zn(s) + h2o(g) →zno(s) + h2(g) is known to be hr 0 = 224 kj and is approximately constant from 920 k up to 1280 k. the standard reaction free energy is +33 kj at 1280 k. calculate the equilibrium constant at 1280 k and then calculate the temperature at which the equilibrium constant becomes greater than 1.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, Vicky22Shz
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1

Chemistry, 22.06.2019 18:30, robjaykay
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 04:40, twinchristiansp4xhd2
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
Do you know the correct answer?
The standard reaction enthalpy of zn(s) + h2o(g) →zno(s) + h2(g) is known to be hr 0 = 224 kj and i...
Questions in other subjects:






Mathematics, 03.07.2019 16:20




Mathematics, 03.07.2019 16:20


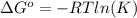
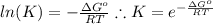
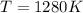 and the ideal gas law constant
and the ideal gas law constant 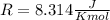 , we obtain:
, we obtain: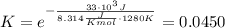
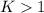 , then
, then 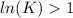 and
and 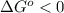 .
.