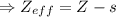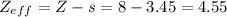Chemistry, 17.12.2019 23:31, Solany6527
Effective nuclear charge, zeff, is defined as:
zeff=z−s
where z is true nuclear charge and s is the amount of shielding.
in 1930, john c. slater devised the following set of empirical rules to estimate s for a designated ns or np electron:
1. write the electron configuration of the element, and group the subshells as follows: (1s), (2s, 2p), (3s, 3p), (3d), (4s, 4p), (4d), (4f ), (5s, 5p), and so on.
2. electrons in groups to the right of the (ns, np) group contribute nothing to the shielding constant for the designated electron.
3. all the other electrons in the (ns, np) group shield the designated electron to the extent of 0.35 each.
4. all electrons in the n−1 shell shield to the extent of 0.85 each.
5. all electrons in the n−2 shell, or lower, shield completely—their contributions to the shielding constant are 1.00 each.
when the designated electron is in an nd or nf group, rules (i), (ii), and (iii) remain the same but rules (iv) and (v) are replaced by the following: each electron in a group lying to the left of the nd or nf group contributes 1.00 to the shielding constant. these rules are a simplified generalization based on the average behavior of different types of electrons.
part a) calculate zeff for a valence electron in an oxygen atom.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, david838843
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3
Do you know the correct answer?
Effective nuclear charge, zeff, is defined as:
zeff=z−s
where z is true nuclear charg...
zeff=z−s
where z is true nuclear charg...
Questions in other subjects:








World Languages, 18.02.2020 23:11

Mathematics, 18.02.2020 23:11


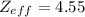
![[Z_{eff}]](/tpl/images/0423/1699/163e1.png) is the net nuclear charge experienced by the electron in a given atom. It is always less than the actual charge of the nucleus [Z], due to shielding by electrons in the inner shells.
is the net nuclear charge experienced by the electron in a given atom. It is always less than the actual charge of the nucleus [Z], due to shielding by electrons in the inner shells.