
Chemistry, 17.12.2019 22:31, missy922527
Consider the following system at equilibrium where h° = -87.9 kj, and kc = 83.3, at 500 k. pcl3(g) + cl2(g) pcl5(g) when 0.17 moles of cl2(g) are added to the equilibrium system at constant temperature: the value of kc the value of qc kc. the reaction must run in the forward direction to restablish equilibrium. run in the reverse direction to restablish equilibrium. remain the same. it is already at equilibrium. the concentration of pcl3 will

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:10, mani1682
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 08:00, mackaylabarnes22
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3

Chemistry, 23.06.2019 09:40, ventalexander8406
Write balanced nuclear equations for the formation of five elements whose atomic number is between helium (2) and iron (26):
Answers: 1
Do you know the correct answer?
Consider the following system at equilibrium where h° = -87.9 kj, and kc = 83.3, at 500 k. pcl3(g) +...
Questions in other subjects:





Social Studies, 18.10.2019 18:00

Advanced Placement (AP), 18.10.2019 18:00





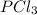 will decrease.
will decrease.




