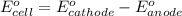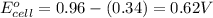Chemistry, 17.12.2019 19:31, Lindseycline123
No−3(aq)+4h+(aq)+3e−→no(g)+2h2o(l)e ∘=0.96v clo2(g)+e−→clo2−(aq)e∘=0.95v cu2+(aq)+2e−→cu(s)e∘=0.34v 2h+(aq)+2e−→h2(g)e∘=0.00v pb2+(aq)+2e−→pb(s)e∘=−0.13v fe2+(aq)+2e−→fe(s)e∘=−0.45v part a use appropriate data to calculate e∘cell for the reaction. 3cu(s)+2no−3(aq)+8h+(aq)→3cu2+(aq)+ 2no(g)+4h2o(l)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, gizmo50245
Calculate the mass percent of hydrogen in methyl acetate
Answers: 1

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2


Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
Do you know the correct answer?
No−3(aq)+4h+(aq)+3e−→no(g)+2h2o(l)e ∘=0.96v clo2(g)+e−→clo2−(aq)e∘=0.95v cu2+(aq)+2e−→cu(s)e∘=0.34v...
Questions in other subjects:

English, 18.01.2022 14:10

World Languages, 18.01.2022 14:10

Mathematics, 18.01.2022 14:10



Mathematics, 18.01.2022 14:20

Geography, 18.01.2022 14:20


English, 18.01.2022 14:20


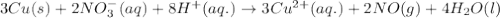
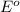 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction.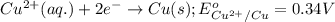 ( × 3 )
( × 3 )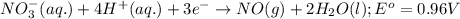 ( × 2 )
( × 2 )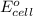 of the reaction, we use the equation:
of the reaction, we use the equation: