
Hich one of the following statements about orbital hybridization is incorrect?
m
a. the carbon atom in ch4 is sp3 hybridized.
b. the carbon atom in co2 is sp hybridized.
c. the nitrogen atom in nh3 is sp2 hybridized.
d. sp2 hybrid orbitals are coplanar, and at 120 to each other.
e. sp hybrid orbitals lie at 180° to each other.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, babygirl2984
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3


Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
Do you know the correct answer?
Hich one of the following statements about orbital hybridization is incorrect?
m
a. the...
m
a. the...
Questions in other subjects:

World Languages, 12.02.2021 14:00

English, 12.02.2021 14:00


Mathematics, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

English, 12.02.2021 14:00


History, 12.02.2021 14:00

History, 12.02.2021 14:00


![\text{Number of electron pair}=\frac{1}{2}[V+N-C+A]](/tpl/images/0422/7584/9e987.png)
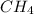
![\text{Number of electrons}=\frac{1}{2}\times [4+4]=4](/tpl/images/0422/7584/b4293.png)
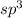 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.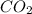
![\text{Number of electrons}=\frac{1}{2}\times [4]=2](/tpl/images/0422/7584/34bd3.png)
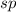 and the electronic geometry of the molecule will be linear.
and the electronic geometry of the molecule will be linear.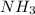
![\text{Number of electrons}=\frac{1}{2}\times [5+3]=4](/tpl/images/0422/7584/5f4d9.png)




