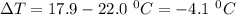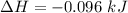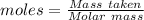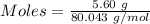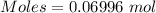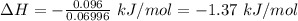Commercial cold packs consist of solid nh4no3 and water. in a coffee-cup calorimeter, 5.60g nh4no3 is dissolved in 100g of water at 22.0c; the temperature falls to 17.9c. assuming that the specific heat capacity of the solution is 4.18 j/(g*k), calculate the enthalpy of dissolution of nh4no3, in kj/mol.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, granthazenp5e9mj
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1
Do you know the correct answer?
Commercial cold packs consist of solid nh4no3 and water. in a coffee-cup calorimeter, 5.60g nh4no3 i...
Questions in other subjects:


Mathematics, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01



Social Studies, 20.09.2020 05:01




Mathematics, 20.09.2020 05:01


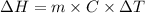
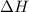 is the enthalpy of dissolution of [tex[NH_4NO_3[/tex]
is the enthalpy of dissolution of [tex[NH_4NO_3[/tex] is the temperature change
is the temperature change