Chemistry, 17.12.2019 04:31, coolquezzie
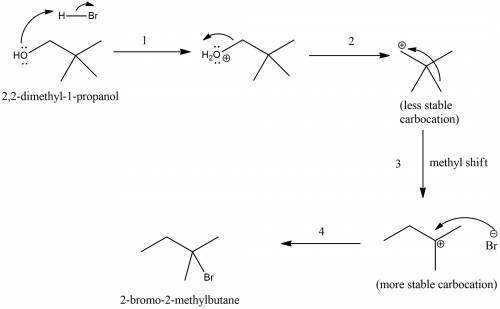
The reaction of 2,2-dimethyl-1-piopanol with hbr is very slow and gives 2-bromo- 2-methyibutane as the major product. give a mechanistic explanation for these observations. select all that apply. stereoelectronic effects result in an antic op lanar rearrangement of the carbon skeleton. steric hindrance prevents nucleophilic attack. the mechanism requites the development of an unstable positively charged species in the transition state. the mechanism results in a carbocation rearrangement in which a methyl shift occurs. the mechanism requires dissociation of a poor leaving group.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1

Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 00:30, natishtaylor1p8dirz
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
Do you know the correct answer?
The reaction of 2,2-dimethyl-1-piopanol with hbr is very slow and gives 2-bromo- 2-methyibutane as t...
Questions in other subjects:

Mathematics, 28.06.2019 15:00


Mathematics, 28.06.2019 15:00

Mathematics, 28.06.2019 15:00


Chemistry, 28.06.2019 15:00




Mathematics, 28.06.2019 15:00


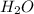 .
.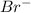 attacks the stable carbocation to produce 2-bromo-2-methylbutane.
attacks the stable carbocation to produce 2-bromo-2-methylbutane.