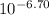Chemistry, 17.12.2019 04:31, Trishamw04
Astudent was given a 50.0 ml of 0.10 m solution of an unknown diprotic acid, h2a, which was titrated with 0.10 m naoh. after a total of 25.0 ml of naoh was added, the resulting solution has a ph of 6.70.
after a total of 50.0 ml of naoh was added, the ph of the solution was 8.00.
determine the values of ka1 and ka2 for the diprotic acid.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
Do you know the correct answer?
Astudent was given a 50.0 ml of 0.10 m solution of an unknown diprotic acid, h2a, which was titrated...
Questions in other subjects:










Mathematics, 06.12.2019 20:31