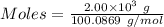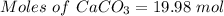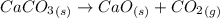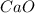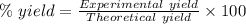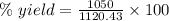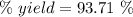Chemistry, 17.12.2019 04:31, lmoleary7466
Quicklime, cao, can be prepared by roasting limestone, caco3, according to the following reaction. caco3(s) ∆→ cao(s) + co2(g). when 2.00 × 103 g caco3 is heated, the actual yield of cao is 1.05 × 103 g. what is the percentage yield?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2


Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
Do you know the correct answer?
Quicklime, cao, can be prepared by roasting limestone, caco3, according to the following reaction. c...
Questions in other subjects:







Mathematics, 02.10.2019 21:30

Spanish, 02.10.2019 21:30


History, 02.10.2019 21:30


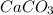 :-
:-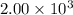 g
g