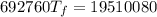Chemistry, 17.12.2019 04:31, aaronjcerrato
A4.00 g sample of a metal (specific heat = 0.600 j g-1°c-1 is heated to 75 degrees celcius and then dropped into 165 g of water in a calorimeter. what is the final temperature of the water if the initial temperature is 28 degrees celcius? the specific heat capacity of water is 4.184 j/g.°c.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, arnold2619
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
Do you know the correct answer?
A4.00 g sample of a metal (specific heat = 0.600 j g-1°c-1 is heated to 75 degrees celcius and then...
Questions in other subjects:

History, 22.05.2020 03:58








Chemistry, 22.05.2020 03:58