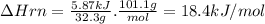Potassium nitrate, kno3, has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 32.3 g of kno3 is dissolved in 243 g of water at 23.00 °c. kno3(s)+h2o(aq) > koh(aq)+hno3(aq)the temperature of the resulting solution decreases to 17.90 °c. assume the resulting solution has the same specific heat as water, 4.184 j/(g·°c), and that there is negligible heat loss to the surroundings.1. how much heat was released by the solution? 2. what is the enthalpy of the reaction?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Do you know the correct answer?
Potassium nitrate, kno3, has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 32.3 g...
Questions in other subjects:

Physics, 26.02.2020 06:39

Mathematics, 26.02.2020 06:39

Mathematics, 26.02.2020 06:39

English, 26.02.2020 06:40

History, 26.02.2020 06:40



Mathematics, 26.02.2020 06:44

Mathematics, 26.02.2020 06:46

Physics, 26.02.2020 06:46