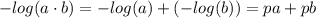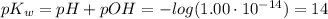Chemistry, 17.12.2019 02:31, Jasmineemarieee
The ph of a solution is the negative logarithm of the molar concentration of hydronium ion, that is, ph=−log[h3o+] in neutral solutions at 25 ∘c, [h3o+]=10−7 m and ph=7. as [h3o+] increases, ph decreases, so acidic solutions have a ph of less than 7. basic solutions have a ph greater than 7. the hydroxide and hydronium ion concentrations are related by the the ion-product constant of water, kw , as follows: kw=1.0×10−14=[h3o+][oh−] in the same way as the ph, we can define the poh as poh=−log[oh−]. it follows from the kw expression that ph+poh=14.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
Do you know the correct answer?
The ph of a solution is the negative logarithm of the molar concentration of hydronium ion, that is,...
Questions in other subjects:










Business, 01.07.2021 22:00


![pH = -log[H^+]](/tpl/images/0421/6601/7d119.png)
![pH = -log[H^+], pK_a = -log(K_a)](/tpl/images/0421/6601/29e90.png) etc.
etc.![[H_3O^+] = [OH^-] = 1.00\cdot 10^{-7} M](/tpl/images/0421/6601/e5313.png)
![pH = -log[H_3O^+] = -log(1.00\cdot 10^{-7}) = 7.00](/tpl/images/0421/6601/b738b.png)
![[H_3O^+] =2.00\cdot 10^{-7} M](/tpl/images/0421/6601/25e55.png)
![pH = -log[H_3O^+] = -log(2.00\cdot 10^{-7}) = 6.70](/tpl/images/0421/6601/deab9.png)
![K_w=[H_3O^+][OH^-]](/tpl/images/0421/6601/16faa.png)
![K_w=[H_3O^+][OH^-]=1.00\cdot10^{-14}](/tpl/images/0421/6601/58549.png)
![-log(K_w)=-log([H_3O^+][OH^-])](/tpl/images/0421/6601/4aed2.png)