

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Do you know the correct answer?
Express the equilibrium constant for the following reaction.2 k(s) + 2 h2o(l) ↔ 2 koh(aq) + h2(g)k =...
Questions in other subjects:

English, 29.04.2021 14:00


Chemistry, 29.04.2021 14:00





Mathematics, 29.04.2021 14:00

Computers and Technology, 29.04.2021 14:00

Mathematics, 29.04.2021 14:00


![K=[KOH]^2[H_2]](/tpl/images/0421/6907/b9417.png)
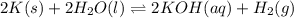
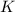 will be,
will be,




