
Chemistry, 17.12.2019 02:31, qmsadler01
The reaction 2nobr (g) → 2 no (g) + br2 (g) is a second-order reaction with a rate constant of 0.80 m-1s-1 at 11 °c. if the initial concentration of nobr is 0.0440 m, the concentration of nobr after 6.0 seconds is

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2



Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
Do you know the correct answer?
The reaction 2nobr (g) → 2 no (g) + br2 (g) is a second-order reaction with a rate constant of 0.80...
Questions in other subjects:


History, 18.11.2020 21:50


Computers and Technology, 18.11.2020 21:50



Social Studies, 18.11.2020 21:50

History, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50


![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0421/6919/ccade.png)
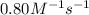
![[A_t]](/tpl/images/0421/6919/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0421/6919/dc622.png) = initial concentration = 0.0440 M
= initial concentration = 0.0440 M![0.80\times 6.0=\frac{1}{[A_t]}-\frac{1}{0.0440}](/tpl/images/0421/6919/0ff8f.png)
![[A_t]=0.036M](/tpl/images/0421/6919/f7d60.png)




