Chemistry, 17.12.2019 01:31, shayla3613
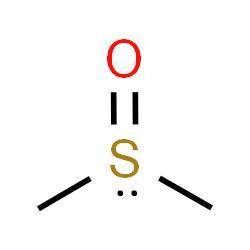
Dimethyl sulfoxide is an important polar aprotic solvent that can dissolve both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. because it penetrates the skin very readily, it is sometimes used as a vehicle for topical application of pharmaceuticals.
draw the structure of dimethyl sulfoxide. include any nonbonding electrons on sulfur, and minimize formal charges by allowing sulfur to expand its octet.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, ian2006huang
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
Do you know the correct answer?
Dimethyl sulfoxide is an important polar aprotic solvent that can dissolve both polar and nonpolar c...
Questions in other subjects:




Mathematics, 02.03.2021 19:50




Mathematics, 02.03.2021 19:50


Chemistry, 02.03.2021 19:50