
Consider the following reaction at equilibrium. what effect will increasing the temperature have on the system? fe3o4(s) + co(g) ↔ 3 feo(s) + co2(g) δh°= +35.9 kjthe equilibrium constant will decrease. no effect will be observed. the reaction will shift to the right in the direction of products. the equilibrium constant will increase. the reaction will shift to the left in the direction of reactants

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
Do you know the correct answer?
Consider the following reaction at equilibrium. what effect will increasing the temperature have on...
Questions in other subjects:


Mathematics, 20.01.2021 16:00


Social Studies, 20.01.2021 16:00



Mathematics, 20.01.2021 16:00



Mathematics, 20.01.2021 16:10


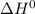 of the given reaction is positive and thus, on increasing temperature, reaction will go in forward direction.
of the given reaction is positive and thus, on increasing temperature, reaction will go in forward direction.



