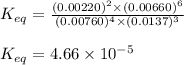9. the first step in industrial nitric acid production is the catalyzed oxidation of ammonia. without a catalyst, a different reaction predominates: 4nh3(g) + 3o2(g) ⇔ 2n2(g) + 6h2o(g) when 0.0120 mol gaseous nh3 and 0.0170 mol gaseous o2 are placed in a 1.00 l container at a certain temperature, the n2 concentration at equilibrium is 2.20×10-3 m. calculate keq for the reaction at this temperature.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 12:00, sophiaa23
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2
Do you know the correct answer?
9. the first step in industrial nitric acid production is the catalyzed oxidation of ammonia. withou...
Questions in other subjects:


Mathematics, 31.01.2020 11:47

Mathematics, 31.01.2020 11:47

English, 31.01.2020 11:47



Mathematics, 31.01.2020 11:47



History, 31.01.2020 11:47


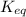 is
is 
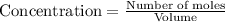
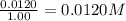
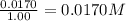
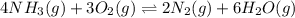


![0.0120-4x=[0.0120-(4\times 0.00110)]=0.00760M](/tpl/images/0421/4577/5e570.png)
![0.0170-3x=[0.0170-(3\times 0.00110)]=0.0137M](/tpl/images/0421/4577/b5ac6.png)
![6x=[6\times 0.00110]=0.00660M](/tpl/images/0421/4577/d0282.png)
![K_{eq}=\frac{[N_2]^2\times [H_2O]^6}{[NH_3]^4\times [O_2]^3}](/tpl/images/0421/4577/7f887.png)