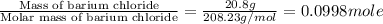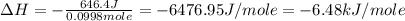Chemistry, 17.12.2019 00:31, raulhill98
An amount of solid barium chloride, 20.8 g, is dissolved in 100 g water in a coffee-cup calorimeter by the reaction: bacl2 (s) ba2+(aq) + 2cl−(aq) the water is originally at 25.0 °c and after the reaction the temperature of the solution is 26.6 °c. (cs = 4.04 j/(g°c) for the solution). what is the enthalpy change (δh) associated with the reaction as written?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1
Do you know the correct answer?
An amount of solid barium chloride, 20.8 g, is dissolved in 100 g water in a coffee-cup calorimeter...
Questions in other subjects:

History, 04.05.2021 20:50

Mathematics, 04.05.2021 20:50

Chemistry, 04.05.2021 20:50


Mathematics, 04.05.2021 20:50




Social Studies, 04.05.2021 20:50

History, 04.05.2021 20:50


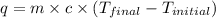
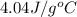
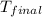 = final temperature =
= final temperature = 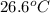
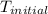 = initial temperature =
= initial temperature = 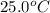
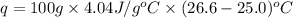
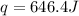
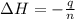
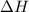 = enthalpy change = ?
= enthalpy change = ?