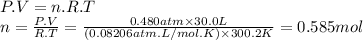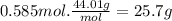Chemistry, 16.12.2019 22:31, cadenhuggins2
Carbon dioxide gas is collected at in an evacuated flask with a measured volume of . when all the gas has been collected, the pressure in the flask is measured to be . calculate the mass and number of moles of carbon dioxide gas that were collected. be sure your answer has the correct number of significant digits.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, isalih7256
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 17:30, ander67061
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
Do you know the correct answer?
Carbon dioxide gas is collected at in an evacuated flask with a measured volume of . when all the ga...
Questions in other subjects:


Business, 27.12.2020 14:00

Mathematics, 27.12.2020 14:00

Mathematics, 27.12.2020 14:00

Biology, 27.12.2020 14:00


Biology, 27.12.2020 14:00

Social Studies, 27.12.2020 14:00


Spanish, 27.12.2020 14:00