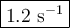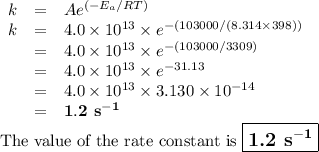Chemistry, 16.12.2019 22:31, caveman171
If the activation energy for a given compound is found to be 103 kj/mol, with a frequency factor of 4.0 × 1013 s-1, what is the rate constant for this reaction at 398 k? 2.5 × 107 s-18.2 s-13.9 × 1010 s-11.2 s-11.7 × 1010 s-1

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:40, wbrandi118
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
Do you know the correct answer?
If the activation energy for a given compound is found to be 103 kj/mol, with a frequency factor of...
Questions in other subjects:


Geography, 10.03.2021 20:00





Mathematics, 10.03.2021 20:00

Mathematics, 10.03.2021 20:00


Mathematics, 10.03.2021 20:00