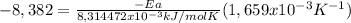Chemistry, 16.12.2019 21:31, krandall232
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate constant of 7.69 m-1s-1 at 219 k. determine the activation energy for this reaction.23.8 kj/mol11.5 kj/mol12.5 kj/mol42.0 kj/mol58.2 kj/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1


Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
Do you know the correct answer?
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate con...
Questions in other subjects:

Mathematics, 09.12.2020 20:00

Computers and Technology, 09.12.2020 20:00

SAT, 09.12.2020 20:00


Mathematics, 09.12.2020 20:00

Social Studies, 09.12.2020 20:00

Health, 09.12.2020 20:00

English, 09.12.2020 20:00