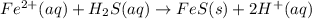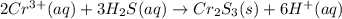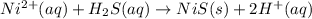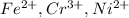Chemistry, 16.12.2019 20:31, genyjoannerubiera
Most sulfide compounds of the transition metals are insoluble in water. many of these metal sulfides have striking and characteristic colors by which we can identify them. therefore, in the analysis of mixtures of metal ions, it is very common to precipitate the metal ions by using dihydrogen sulfate (commonly called hydrogen sulfide), h2s. suppose you had a mixture of fe2 , cr3 , and ni2 . complete the net ionic equations for the precipitation of these metal ions by the use of h2s. (type your answers using the format fe2 for fe2 .)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1


Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
Do you know the correct answer?
Most sulfide compounds of the transition metals are insoluble in water. many of these metal sulfides...
Questions in other subjects:



English, 03.07.2019 15:30


Mathematics, 03.07.2019 15:30

Mathematics, 03.07.2019 15:30

Spanish, 03.07.2019 15:30


Chemistry, 03.07.2019 15:30