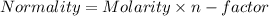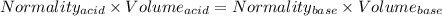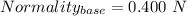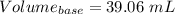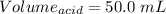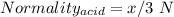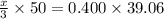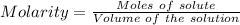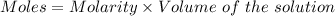Chemistry, 16.12.2019 19:31, secondcolinmills005
You are given a 1.00 g sample of an unknown tri-protic acid, which you dissolve in 50.0 ml of water containing phenolphthalein indicator. you titrate the acid solution with standardized 0.400 m koh(aq). it requires 39.06 ml of the koh solution to produce a light pink indicator color. what is the molecular weight of the unknown acid?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, akatsionis25
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 07:30, eburnhisel2023
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
Do you know the correct answer?
You are given a 1.00 g sample of an unknown tri-protic acid, which you dissolve in 50.0 ml of water...
Questions in other subjects:

History, 11.12.2021 09:50

Mathematics, 11.12.2021 09:50


Mathematics, 11.12.2021 09:50



Geography, 11.12.2021 09:50