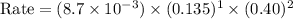Chemistry, 16.12.2019 19:31, pearpeaerrr1993
Consider the following reaction between mercury(ii) chloride and oxalate ion: 2hgcl2(aq)+c2o2−4(aq)→2cl−(aq)+2co2 (g)+hg2cl2(s) the initial rate of this reaction was determined for several concentrations of hgcl2 and c2o2−4, and the following rate data were obtained for the rate of disappearance of c2o2−4:

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 02:50, giavanleer14
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 04:00, Mitchmorgan3816
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1

Chemistry, 23.06.2019 06:30, aurikmah2005
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1

Chemistry, 23.06.2019 12:30, Vipain02
The equilibrium constant kc for the reaction 2 nocl(g) → 2 no(g) + cl2(g) is 0.453 at a certain temperature. a mixture of nocl, no, and cl2 with concentrations 1.30, 1.20, and 0.600 m, respectively, was introduced into a container at this temperature. which of the following is true? 1. no apparent reaction takes place. 2. [cl2] = 0.30 m at equilibrium. 3. nocl(g) is produced until equilibrium is reached. 4. [nocl] = [no] = [cl2] at equilibrium. 5. cl2(g) is produced until equilibrium is
Answers: 3
Do you know the correct answer?
Consider the following reaction between mercury(ii) chloride and oxalate ion: 2hgcl2(aq)+c2o2−4(aq)...
Questions in other subjects:


History, 04.09.2020 22:01

History, 04.09.2020 22:01




Health, 04.09.2020 22:01

Mathematics, 04.09.2020 22:01

Mathematics, 04.09.2020 22:01

English, 04.09.2020 22:01



 and
and  , and the following rate data were obtained for the rate of disappearance of
, and the following rate data were obtained for the rate of disappearance of 
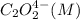 Rate (M/s)
Rate (M/s)
![\text{Rate}=k[HgCl_2]^a[C_2O_2^{4-}]^b](/tpl/images/0420/9839/2a902.png)
 ....(1)
....(1)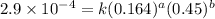 ....(2)
....(2)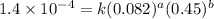 ....(3)
....(3) ....(4)
....(4)
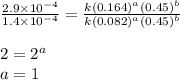
![\text{Rate}=k[HgCl_2]^1[C_2O_2^{4-}]^2](/tpl/images/0420/9839/2292e.png)


 is 0.40 M.
is 0.40 M.