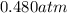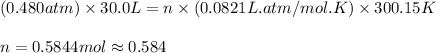Chemistry, 16.12.2019 18:31, Thejollyhellhound20
Carbon dioxide gas is collected at 27.0(degrees)c in an evacuated flask with a measured volume of 30.0l. when all the gas has been collected, the pressure in the flask is measured to be 0.480atm.
calculate the mass and number of moles of carbon dioxide gas that were collected. round your answer to 3 significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2



Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1
Do you know the correct answer?
Carbon dioxide gas is collected at 27.0(degrees)c in an evacuated flask with a measured volume of 30...
Questions in other subjects:


Mathematics, 10.12.2019 11:31


English, 10.12.2019 11:31



English, 10.12.2019 11:31


Biology, 10.12.2019 11:31