
Chemistry, 14.12.2019 01:31, orlando19882000
Calculate the ph of a 0.208 0.208 m solution of ethylenediamine ( h 2 nch 2 ch 2 nh 2 h2nch2ch2nh2). the p k a pka values for the acidic form of ethylenediamine ( h + 3 nch 2 ch 2 nh + 3 h3+nch2ch2nh3+) are 6.848 6.848 ( p k a1 pka1) and 9.928 9.928 ( p k a2 pka2). ph = ph= calculate the concentration of each form of ethylenediamine in this solution at equilibrium.
[ h 2 nch 2 ch 2 nh 2 ] =
[h2nch2ch2nh2]= m m
[ h 2 nch 2 ch 2 nh + 3 ] =
[h2nch2ch2nh3+]= m m
[ h + 3 nch 2 ch 2 nh + 3 ] =
[h3+nch2ch2nh3+]=

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3


Chemistry, 23.06.2019 14:30, maelonramirez
William has eight more nickels than dimes in his pocket for a total of $2.50. which equation could be used to determine the number of x dimes in his pocket?
Answers: 1
Do you know the correct answer?
Calculate the ph of a 0.208 0.208 m solution of ethylenediamine ( h 2 nch 2 ch 2 nh 2 h2nch2ch2nh2)....
Questions in other subjects:

Biology, 10.07.2019 17:30


Social Studies, 10.07.2019 17:30

Social Studies, 10.07.2019 17:30


History, 10.07.2019 17:30





![[H_{2}NCH_{2}NCH_{2}NH_{2}]](/tpl/images/0417/9676/3fc2a.png) = 0.204 M;
= 0.204 M;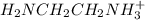 =
= 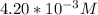
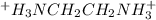 =
= 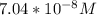
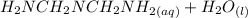 ⇔
⇔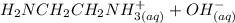
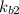 equation (1)
equation (1)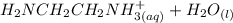 ⇔
⇔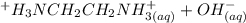
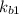 equation (2)
equation (2)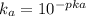
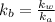

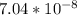
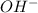 = x
= x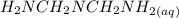 = 0.208-x
= 0.208-x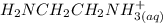 = x
= x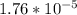
![[H_{2}NCH_{2}NCH_{2}NH_{2(aq)}]](/tpl/images/0417/9676/0b733.png) = 0.208 -
= 0.208 - 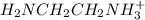
 = 2.38
= 2.38



