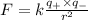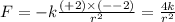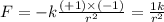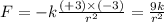Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
Do you know the correct answer?
Choose the compound below that should have the highest melting point according to the ionic bonding...
Questions in other subjects:


Mathematics, 22.04.2020 22:08


Mathematics, 22.04.2020 22:08

English, 22.04.2020 22:08

Mathematics, 22.04.2020 22:08


Mathematics, 22.04.2020 22:08


Physics, 22.04.2020 22:08