
Consider the following system at equilibrium where \delta h° = 18.8 kj, and kc = 9.52×10-2, at 350 k.
ch4(g) + ccl4(g) \rightleftharpoons 2ch2cl2(g)
when 0.31 moles of ccl4(g) are removed from the equilibrium system at constant temperature:
1. the value of
a. increases.
b. decreases.
c. remains the same.
2. the value of qc
a. is greater than
b. is equal to kc.
c. is less than kc.
3. the reaction :
a. run in the forward direction to reestablish equilibrium.
b. run in the reverse direction to reestablish equilibrium.
c. remain the same. it is already at equilibrium.
4. the concentration of ch4 will:
a. increase.
b. decrease.
c. remain the same.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2


Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
Do you know the correct answer?
Consider the following system at equilibrium where \delta h° = 18.8 kj, and kc = 9.52×10-2, at 350 k...
Questions in other subjects:

History, 21.11.2019 05:31


Mathematics, 21.11.2019 05:31






Mathematics, 21.11.2019 05:31


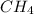 will increase because product will be converted to reactants to reestablish equilibrium.
will increase because product will be converted to reactants to reestablish equilibrium.



