Classify each of the following reactions as one of the four possible types:
1. spontan...

Chemistry, 13.12.2019 22:31, jose0765678755
Classify each of the following reactions as one of the four possible types:
1. spontaneous at all temperatures;
2. nonspontaneous at all temperatures;
3. spontaneous at low t; nonspontaneous at high t;
4. spontaneous at high t; nonspontaneous at low t.
(a) n2(g)+3f2(g)→2nf3(g); δh∘=−249kj; δs∘=−278j/k
(b) n2(g)+3cl2(g)→2ncl3(g); δh∘=460kj; δs∘=−275j/k
(c) n2f4(g)→2nf2(g); δh∘=85kj; δs∘=198j/k

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, kev71
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Biology, 03.12.2021 21:50

Mathematics, 03.12.2021 21:50



Biology, 03.12.2021 21:50


Mathematics, 03.12.2021 21:50

Geography, 03.12.2021 21:50


English, 03.12.2021 21:50


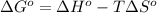



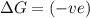 (at low Temperature)
(at low Temperature)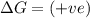 (at high Temperature)
(at high Temperature)








