
Chemistry, 13.12.2019 21:31, dornauriel
Two bulbs are connected by a stopcock. the large bulb, with a volume of 6.00 l, contains nitric oxide at a pressure of 0.700 atm, 0.700 atm, and the small bulb, with a volume of 1.50 l, contains oxygen at a pressure of 2.50 atm. the temperature at the beginning and the end of the experiment is 22 ∘ c 22∘c . after the stopcock is opened, the gases mix and react. 2 no ( g ) + o 2 ( g ) → 2 no 2 ( g ) 2no(g)+o2(g)→2no2(g)
which gases are present at the end of the experiment?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 05:00, lifeoflashay1659
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
Do you know the correct answer?
Two bulbs are connected by a stopcock. the large bulb, with a volume of 6.00 l, contains nitric oxid...
Questions in other subjects:


Advanced Placement (AP), 23.03.2021 04:50







Mathematics, 23.03.2021 04:50


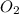 and
and 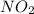
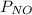 = 0
= 0



