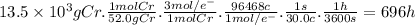Purification of chromium can be achieved by electrorefining chromium from an impure chromium anode onto a pure chromium cathode in an electrolytic cell. how many hours will it take to plate 13.5 kg of chromium onto the cathode if the current passed through the cell is held constant at 30.0 a ? assume the chromium in the electrolytic solution is present as cr 3 +.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1


Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2
Do you know the correct answer?
Purification of chromium can be achieved by electrorefining chromium from an impure chromium anode o...
Questions in other subjects:

Biology, 22.07.2019 11:30









History, 22.07.2019 11:30