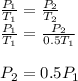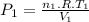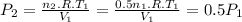Chemistry, 13.12.2019 19:31, gstinson98
Assume that you have a cylinder with a movable piston. what would happen to the gas pressure inside the cylinder if you do the following? part a: decrease the volume to one-fourth the original volume while holding the temperature constant. express your answer in terms of the variable p initial. part b: reduce the kelvin temperature to half its original value while holding the volume constant. express your answer in terms of the variable p initial .part c: reduce the amount of gas to half while keeping the volume and temperature constant. express your answer in terms of the variable p initial .

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Do you know the correct answer?
Assume that you have a cylinder with a movable piston. what would happen to the gas pressure inside...
Questions in other subjects:

Mathematics, 13.04.2021 16:00

History, 13.04.2021 16:00

Mathematics, 13.04.2021 16:00

Mathematics, 13.04.2021 16:00

Social Studies, 13.04.2021 16:00

History, 13.04.2021 16:00



Mathematics, 13.04.2021 16:00

Mathematics, 13.04.2021 16:00