Chemistry, 13.12.2019 06:31, antonjas001
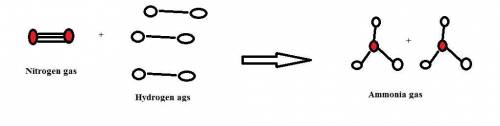
1. how many molecules of ammonia can be created when four molecules of nitrogen are combined with four molecules of hydrogen? include a drawing of the molecules in your answer.
2. what ratio of nitrogen and hydrogen molecules would result in no left-over reactants? explain your answer
3. if 100.0g of nitrogen is reacted with 100.0g of hydrogen, what is the theoretical yield of the reaction? what is the excess reactant? what is the limiting reactant?
me i'm so confused

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, danielhall
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3


Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
Do you know the correct answer?
1. how many molecules of ammonia can be created when four molecules of nitrogen are combined with fo...
Questions in other subjects:

English, 27.07.2021 17:10



Mathematics, 27.07.2021 17:10

Mathematics, 27.07.2021 17:10





Mathematics, 27.07.2021 17:10



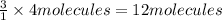 of hydrogen gas.
of hydrogen gas.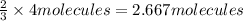 of ammonia
of ammonia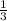 = 1 : 3
= 1 : 3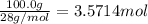
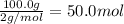
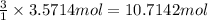 of hydrogen gas.
of hydrogen gas.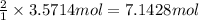 of ammonia
of ammonia