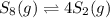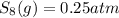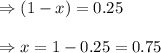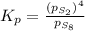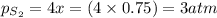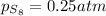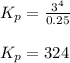1.
a sample of s8 (8) is placed in an otherwise empty rigid container at 1325 k at an in...

Chemistry, 13.12.2019 06:31, cerlos110484
1.
a sample of s8 (8) is placed in an otherwise empty rigid container at 1325 k at an initial pressure of 1.00 atm, where it
decomposes to s2 by the reaction: s8() 4 s2(2). |
at equilibrium, the partial pressure of s, is 0.25 atm. calculate k, for this reaction at 1325 k

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, coryoddoc3685
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 20.10.2020 03:01


Mathematics, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01


Mathematics, 20.10.2020 03:01

Biology, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01


Mathematics, 20.10.2020 03:01


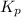 is 324
is 324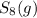 = 1.00 atm
= 1.00 atm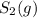 follows:
follows: