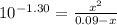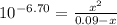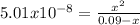Phosphorous acid, h3po3(aq), is a diprotic oxyacid that is an important compound in industry and agriculture. k pka1 k pka2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.8 m h3po3(aq) with 1.8 m koh(aq). before addition of any koh: after addition of 25.0 ml koh: after addition of 50.0 ml koh: after addition of 75.0 ml koh: after addition of 100.0 ml koh:

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 00:30, cashkidd2200
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Do you know the correct answer?
Phosphorous acid, h3po3(aq), is a diprotic oxyacid that is an important compound in industry and agr...
Questions in other subjects:

Arts, 11.03.2021 21:30


Mathematics, 11.03.2021 21:30

Health, 11.03.2021 21:30

Mathematics, 11.03.2021 21:30


Arts, 11.03.2021 21:30

English, 11.03.2021 21:30