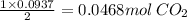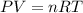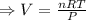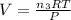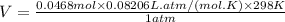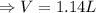Chemistry, 13.12.2019 04:31, redbeast677
The following is the balanced equation of the combustion of methane gas with oxygen gas:
ch4(g) + 2o2(g) -> co2(g) + 2h2o(l)
if you ran a reaction in a sealed balloon at 25o c with 1 g of methane, ch4, and 3 g of oxygen, o2, what is the end volume of the balloon once the reaction is complete? the pressure was constant at 1 atm. assume all gases behave ideally and you have 100% yield. (ignore vapor pressure of water)
a. 4.9 l
b. 0.096 l
c. 1.1 l
d 1.5 l
e. 0.13 l

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 01:30, elizediax8683
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1
Do you know the correct answer?
The following is the balanced equation of the combustion of methane gas with oxygen gas:
Questions in other subjects:

Mathematics, 01.07.2021 09:10




Mathematics, 01.07.2021 09:10



English, 01.07.2021 09:20

Arts, 01.07.2021 09:20

English, 01.07.2021 09:20


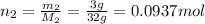
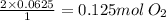 .
.