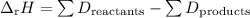Chemistry, 13.12.2019 03:31, mikayla843
Calculate the average bond energy of the sulfur-oxygen bonds in so2 (in kj/mol), given the following information. sf4(g) + 2 h2o(g) → so2(g) + 4 hf(g) δh = –123 kj bond dissociation energies: s–f 327 kj/mol f–f 154 kj/mol h–f 565 kj/mol h–o 467 kj/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 23.06.2019 08:30, aaliyahnv07
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
Do you know the correct answer?
Calculate the average bond energy of the sulfur-oxygen bonds in so2 (in kj/mol), given the following...
Questions in other subjects:

Mathematics, 28.07.2019 15:00





Biology, 28.07.2019 15:00