
Chemistry, 13.12.2019 03:31, bentonknalige
Suppose 0.245 g of sodium chloride is dissolved in 50. ml of a 18.0 m m aqueous solution of silver nitrate.
calculate the final molarity of chloride anion in the solution. you can assume the volume of the solution doesn’t change when the sodium chloride is dissolved in it.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
Do you know the correct answer?
Suppose 0.245 g of sodium chloride is dissolved in 50. ml of a 18.0 m m aqueous solution of silver n...
Questions in other subjects:


Mathematics, 16.04.2020 20:57

History, 16.04.2020 20:57

Biology, 16.04.2020 20:57




Mathematics, 16.04.2020 20:57

History, 16.04.2020 20:57

History, 16.04.2020 20:57


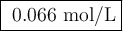
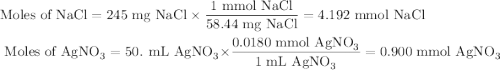
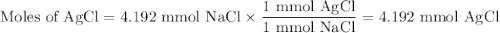
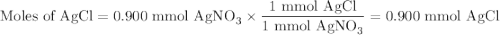
![\text{[Cl$^{-}$] } = \dfrac{\text{3.292 mmol}}{\text{50. mL}} = \textbf{0.066 mol/L}\\\text{The concentration of chloride ion is $\large \boxed{\textbf{0.066 mol/L}}$}](/tpl/images/0416/3996/2071d.png)




