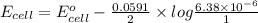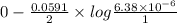Chemistry, 13.12.2019 02:31, tmgoddess2004
The ksp of copper(ii) ferrocyanide (cu2[fe(cn)6]) is 1.3 × 10−16 at 25°c. determine the potential of a concentration cell in which one half-cell consists of a copper electrode in 1.00 m copper(ii) nitrate, and the other consists of a copper electrode in a saturated solution of cu2[fe(cn)6].
ferrocyanide, ([fe(cn)6]4−), is a complex ion.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:10, bossboybaker
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2
Do you know the correct answer?
The ksp of copper(ii) ferrocyanide (cu2[fe(cn)6]) is 1.3 × 10−16 at 25°c. determine the potential of...
Questions in other subjects:





Mathematics, 31.07.2021 03:50


English, 31.07.2021 03:50


English, 31.07.2021 03:50


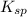 of the given reaction is as follows.
of the given reaction is as follows.![K_{sp} = [Cu^{2+}]^{2}[Fe(CN)_{6}]](/tpl/images/0416/3120/586ea.png)
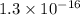 .
.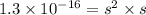
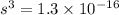
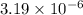
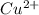 will be calculated as follows.
will be calculated as follows.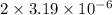
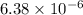 M
M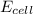 as follows.
as follows.