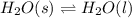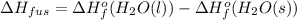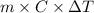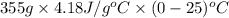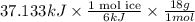Use the standard enthaplies of formation to calculate the standard change in enthaply for the melting of ice. (-291.8 kj/mol for h20 (s). use this value to calculate the mass of ice required to cool 355 ml of a beverage from the room temperature (25 degree celsius) to 0 degree celsius. assume that the specfic heat capacity and the density of the beverage are the same as those of water.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 12:30, dependentclause5828
Idid a lab for chemistry where we put nails in a copper (ii) chloride solution. 1. why did the reaction stop? which reactant was used up? how do you know? 2. describe what was happening to the atoms of iron and copper during the reaction. what is this type of reaction called? 3. what would happen to the ratio of copper to iron if you had placed more nails in the beaker? if you had let the reaction go for less time? 4. what is the accepted ratio of copper atoms to iron atoms in this reaction? account for differences between your experimental value and the accepted value. write the balanced equation for the reaction.
Answers: 2

Chemistry, 23.06.2019 14:30, daphnevlogs11
If energy was included in a chemical reaction, on which side of the equation would it be written for an endothermic reaction?
Answers: 1

Chemistry, 23.06.2019 19:40, texas101st78
Alab technician needs to create 570.0 milliliters of a 2.00 m solution of magnesium chloride (mgcl2). to make this solution, how many grams of magnesium chloride does the technician need? refer to the periodic table for . express your answer to three significant figures.
Answers: 3
Do you know the correct answer?
Use the standard enthaplies of formation to calculate the standard change in enthaply for the meltin...
Questions in other subjects: