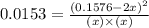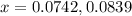Nitrogen and oxygen do not react appreciably at room temperature, as illustrated by our atmosphere. but at high temperatures, the reaction below can proceed to a measurable extent. n2(g) + o2(g) ⇔ 2 no(g) at 3000 k, the reaction above has keq = 0.0153. if 0.3152 mol of pure no is injected into an evacuated 2.0-l container and heated to 3000k, what will be the equilibrium concentration of no?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1
Do you know the correct answer?
Nitrogen and oxygen do not react appreciably at room temperature, as illustrated by our atmosphere....
Questions in other subjects:

Chemistry, 09.06.2020 18:57



Mathematics, 09.06.2020 18:57



English, 09.06.2020 18:57

English, 09.06.2020 18:57



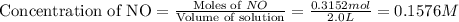
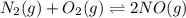
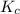 will be,
will be,![K_c=\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0414/9499/71f8f.png)