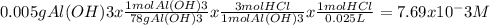Chemistry, 12.12.2019 05:31, nathanscastr02
Antacids are compounds, usually bases, used to decrease the amount of hydrochloric acid in the stomach. write a brief titration procedure for determining how many moles of acid a single antacid tablet would neutralize. this procedure should be detailed and correct so that someone could go into a laboratory and follow the procedure you have written.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3


Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
Do you know the correct answer?
Antacids are compounds, usually bases, used to decrease the amount of hydrochloric acid in the stoma...
Questions in other subjects:

Mathematics, 04.11.2020 03:10

History, 04.11.2020 03:10

Mathematics, 04.11.2020 03:10

Physics, 04.11.2020 03:10

Mathematics, 04.11.2020 03:10

Social Studies, 04.11.2020 03:10

Mathematics, 04.11.2020 03:10


French, 04.11.2020 03:10