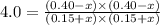Chemistry, 12.12.2019 03:31, antoniaannswiney
Acetic acid, ch3co2h, reacts with ethanol, c2h5oh, to form water and ethyl acetate, ch3co2c2h5. the equilibrium constant for this reaction with dioxane as a solvent is 4.0. what are the equilibrium concentrations for a mixture that is initially 0.15 m in ch3co2h, 0.15 m in c2h5oh, 0.40 m in ch3co2c2h5, and 0.40 m in h2o?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:40, wbrandi118
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 05:30, khaylaperry
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
Do you know the correct answer?
Acetic acid, ch3co2h, reacts with ethanol, c2h5oh, to form water and ethyl acetate, ch3co2c2h5. the...
Questions in other subjects:




English, 12.08.2020 05:01





Mathematics, 12.08.2020 05:01


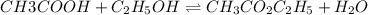
![K_c=\frac{[CH_3CO_2C_2H_5][H_2O]}{[CH_3COOH][C_2H_5OH]}](/tpl/images/0414/6294/2612b.png)