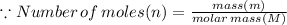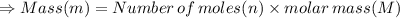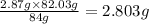Chemistry, 12.12.2019 03:31, jaylynomalley
In the stoichiometry laboratory experiment you reacted sodium bicarbonate with acetic acid to form sodium acetate, water and carbon dioxide according to the following equation. nahco3 + ch3cooh → h2o + co2 + nach3co2 calculate the theoretical yield of the reaction if you reacted 2.87 grams of sodium bicarbonate with sufficient acetic acid to produce sodium acetate. group of answer choices

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, clairajogriggsk
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
Do you know the correct answer?
In the stoichiometry laboratory experiment you reacted sodium bicarbonate with acetic acid to form s...
Questions in other subjects:

Mathematics, 05.05.2021 18:40


English, 05.05.2021 18:40



Mathematics, 05.05.2021 18:40


Mathematics, 05.05.2021 18:40

Mathematics, 05.05.2021 18:40