Chemistry, 12.12.2019 03:31, ayoismeisjjjjuan
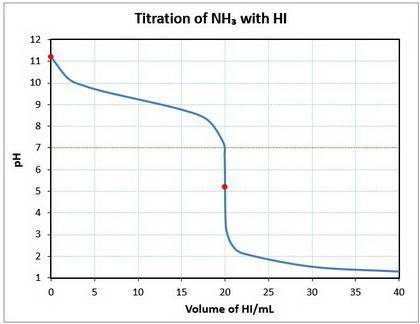
10. a 20.00 ml sample of 0.150 mol/l ammonia (nh3(aq)) is titrated to the equivalence point by 20.0 ml of a solution of 0.150 mol/l of the strong acid hydroiodic acid (hi (
a) write a balanced equation for the titration reaction.
b) what is the ph of the ammonia solution before the titration begins?
c) what is the ph at the equivalence point?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 22.06.2019 10:30, mv603177
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
Do you know the correct answer?
10. a 20.00 ml sample of 0.150 mol/l ammonia (nh3(aq)) is titrated to the equivalence point by 20.0...
Questions in other subjects:



Health, 20.11.2020 07:10


Mathematics, 20.11.2020 07:10

Mathematics, 20.11.2020 07:10




Mathematics, 20.11.2020 07:10




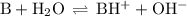
![K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.150 - x} = 1.8 \times 10^{-5}](/tpl/images/0414/6924/2256c.png)
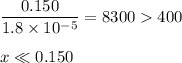
![\dfrac{x^{2}}{0.150} = 1.8 \times 10^{-5}\\\\x^{2} = 0.150 \times 1.8 \times 10^{-5}\\x^{2} = 2.7 \times 10^{-6}\\x = \sqrt{2.7 \times 10^{-6}}\\x = \text{[OH]}^{-} = 1.64 \times 10^{-3} \text{ mol/L}](/tpl/images/0414/6924/0b94c.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(1.64 \times 10^{-3}) = 2.78\\\\\text{pH} = 14.00 - \text{pOH} = 14.00 - 2.78 = \mathbf{11.22}\\\\\text{The pH of the solution at equilibrium is } \large \boxed{\mathbf{11.22}}](/tpl/images/0414/6924/f4986.png)
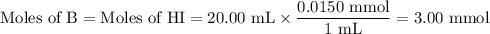
![\rm [BH^{+}] = \dfrac{\text{3.00 mmol}}{\text{40.00 mL}} = \text{0.0750 mol/L}](/tpl/images/0414/6924/607b2.png)
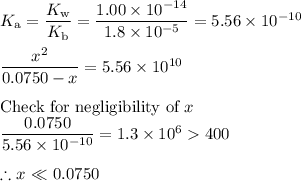
![\dfrac{x^{2}}{0.0750} = 5.56 \times 10^{-10}\\\\x^{2} = 0.0750 \times 5.56 \times 10^{-10}\\x^{2} = 4.17 \times 10^{-11}\\x = \sqrt{4.17 \times 10^{-11}}\\\rm [H_{3}O^{+}] =x = 6.46 \times 10^{-6}\, mol \cdot L^{-1}](/tpl/images/0414/6924/dc4d9.png)
![\text{pH} = -\log{\rm[H_{3}O^{+}]} = -\log{6.46 \times 10^{-6}} = \large \boxed{\mathbf{5.19}}](/tpl/images/0414/6924/b2d28.png)