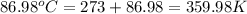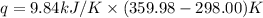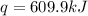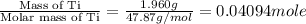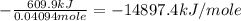Chemistry, 11.12.2019 23:31, cheyennemitchel2680
When 1.960 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 °c to 86.98 °c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3


Chemistry, 23.06.2019 08:00, mshields1994
Amechanical wave that transports a lot of energy will have a
Answers: 2
Do you know the correct answer?
When 1.960 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter incr...
Questions in other subjects:




Biology, 14.07.2019 15:00


Mathematics, 14.07.2019 15:00

Mathematics, 14.07.2019 15:00

History, 14.07.2019 15:00

Mathematics, 14.07.2019 15:00


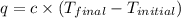
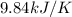
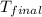 = final temperature =
= final temperature = 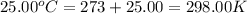
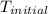 = initial temperature =
= initial temperature =